Abstract
Background: Fatigue remains one of the most severe and disabling symptoms experienced by patients with myeloproliferative neoplasms (MPNs) resulting in impaired social, role, and physician functioning and reduced quality of life (Cancer 2007). Current NCCN Clinical Practice Guidelines in Oncology recommend consideration of use of psychostimulants (PSs) if other causes of fatigue have been ruled out. In this analysis, we evaluate the role of pharmacologic PSs in this population utilizing data from a previously conducted a survey regarding fatigue in MPN patients (Cancer 2016).
Methods: A 70-item internet-based survey was developed by a multidisciplinary team of MPN investigators, patients, and patient advocates that included questions on medication PS use. The survey was promoted online via multiple MPN-related websites during late February to March of 2014. Survey data was immediately captured, downloaded, and stored on secure servers at the Mayo Clinic Survey Research Center. Success of PSs was patient-reported on a 5-level tiered response range from not at all successful to very successful. Categorical variables were compared using chi-square tests, continuous variables using T-tests, and Pearson's rho estimated correlations. P values < 0.05 were considered statistically significant.
Results: Respondents : 1788 MPN patients participated in the online survey. Of these, 1748 consented to the survey and 1676 were included for completing 10 or more survey questions. 116 patients (8.4%) of the 1377 patients who responded to the question regarding PS use reported use of these medications. Use among MPN subtypes varied (9.9% of essential thrombocythemia patients, 9.1% of polycythemia vera patients, and 5.8% of myelofibrosis patients).
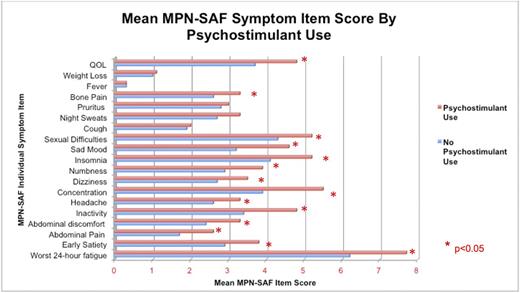
Characteristics : Females used PSs more frequently than males (9.9% females vs 5.4% males, P= 0.006). Use of PSs was most often observed among individuals with severe fatigue (mean brief fatigue inventory (BFI) score 5.7 among those using PSs versus 4.4 among those not using, P < 0.001). Individuals who reported that fatigue impaired sleep were more often PS users (12% of users had impaired sleep versus 6% of users without impaired sleep, P < 0.001). Patients who used PSs were also more symptomatic for the 10 most MPN-specific symptoms assessed via the MPN Total Symptom Score (MPN-TSS; mean 36.3 vs 28.4, P < 0.001). Individual MPN Symptom Assessment Form (MPN-SAF) item scores are shown in Figure 1.
Efficacy : We then evaluated the characteristics of PS users by their reported success in reducing fatigue (102/166). Success in reducing fatigue was reported more often in older respondents (mean age of 58.9 years in those reporting that PSs were somewhat to very helpful vs 53.9 years for not helpful, P = 0.03). Although not significant, patients who reported success in reducing fatigue tended to have a diagnosis of PV (somewhat or very successful in 34/45 for PV; 7/13 for MF, 21/42 for ET, P= 0.05) or female (somewhat or very successful in 54/80 vs 9/20 for male, P= 0.06).
Correlates : Of PS users who completed the secondary portion on fatigue reduction success level (n=102), there was no apparent correlation between MPN-TSS (0-10) and reported success in reduction of fatigue (rho = -0.1; P= 0.5).
Conclusions: Medication PSs are utilized in a notable proportion of MPN patients. MPN patients who use PSs often had significantly worse fatigue than their peers, which may reflects the population in which PSs are most likely to be of suspected benefit. Given the severity of fatigue in this population and the lack of effective fatigue-alleviating strategies, more studies regarding the efficacy of PSs are needed.
Harrison: CTI: Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Shire: Speakers Bureau; Celgene: Consultancy. Mesa: Celgene Corporation: Research Funding; Ariad: Consultancy; Promedico: Research Funding; Incyte Corporation: Research Funding; Galena Biopharma, Inc.: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy; CTI BioPharma Corp.: Research Funding; Gilead Sciences, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal